History of Nuclear Weapons

The history of nuclear weapons chronicles the development of neclear weapons - devices of enormous destructive potential which derive their energy from nuclear fission or nuclear fusion reactions - starting with the scientific breakthroughs of the 1930s which made their development possible, continuing through the nuclear arms race and nuclear testing of the Cold War, and finally with the questions of proliferation and possible use for terrorism in the early 21st century.
Introduction
(U) The first fission weapons ("atomic bombs") were developed in the United States during World War II in what was called the Manhattan Project, at which point two were dropped on Japan. The Soviet Union started development shortly thereafter with their own atomic bomb project, and not long after that both countries developed even more powerful fusion weapons ("hydrogen bombs"). During the Cold War, these two countries each acquired nuclear weapons arsenals numbering in the thousands, placing many of them onto rockets which could hit targets anywhere in the world. Currently there are at least nine countries with functional nuclear weapons. A considerable amount of international negotiating has focused on the threat of nuclear warfare and the proliferation of nuclear weapons to new nations or groups. There have been (at least) four major false alarms, the most recent in 1995, that almost resulted in the US or Russia launching its weapons in retaliation for a supposed attack.
Physics and Politics in the 1930s
(U) In the first decades of the twentieth century, physics was revolutionized with developments in the understanding of the nature of atoms. In 1898 , French physicist Pierre Curie and his Polish wife Marie had discovered that present in pitchblende an ore of uranium was a substance which emitted lar e amounts of radioactivity, which they named radium. This raised the hopes of both scientists and lay people that the elements around us could contain tremendous amounts of unseen energy, waiting to be tapped.
(U) Experiments by Ernest Rutherford in 1911 indicated that the vast majority of an atom's mass was contained in a very small nucleus at its core, made up of protons, surrounded by a web of whirring electrons. In 1932, James Chadwick discovered that the nucleus contained another fundamental particle, the neutron, and in the same year John Cockcroft and Ernest Walton "split the atom" for the first time, the first occasion on which an atomic nucleus of one element had been successfully changed to a different nucleus by artificial means.
(U) In 1934 , French physicists Irene and Frederic Joliot-Curie discovered that artificial radioactivity could be induced in stable elements by bombarding them with alpha particles, and in the same year Italian physicist Enrico Fermi reported similar results when bombarding uranium with neutrons.
(U) In 1938 , Germans Otto Hahn and Fritz Strassmann released the results of their finding proving that what Fermi had witnessed in 1934 was no less than the bursting of the uranium nucleus: nuclear fission. Immediately afterwards, Lise Meitner and Otto Robert Frisch described the theoretical mechanisms of fission and revealed that large amounts ofbinding energy were released in the process. Hungarian Leo Szihird confirmed with his own experiments that along with energy, neutrons were given off in the reaction as well, creating the possibility of a nuclear chain reaction, whereby each fission created two or more other fissions, exponentially releasing energy.
(U) As the Nazi army marched into first Czechoslovakia in 1938, and then Poland in 1939 , officially beginning World War 11, many of Europe's top physicists had already begun to flee from the imminent conflict. Scientists on both sides of the conflict were well aware of the possibility of utilizing nuclear fission as a weapon, but at the time no one was quite sure how it could be done. In the early years of the Second World War, physicists abruptly stopped publishing on the topic of fission, an act of self-censorship to keep the opposing side from gaining any advantages.
From Los Alamos to Hiroshima

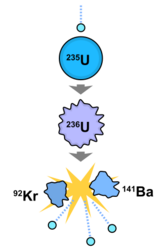
(U) By the beginning of World War II, there was concern among scientists in the Allied nations that Nazi Germany might have their own project to develop fission-based weapons. Organized research first began in Britain as part of the "Tube Alloys" project, and in the United States a small amount of funding was given for research into uranium weapons starting in 1939 with the Uranium Committee under Lyman James Briggs. At the urging of British scientists, though, who had made crucial calculations indicating that a fission weapon could be completed within only a few years, by 1941 the project had been wrested into better bureaucratic hands, and in 1942 carne under the auspices of General Leslie Groves as the Manhattan Project. Scientifically led by the American physicist Robert Oppenheimer, the project brought together the top scientific minds of the day (many exiles from Europe) with the production power of American industry for the goal of producing fission-based explosive devices before Germany could. Britain and the U.S. agreed to pool their resources and information for the project, but the other Allied power - the Soviet Union under Joseph Stalin- was not informed. A massive industrial and scientific undertaking, the Manhattan Project involved many of the world's great physicists in the scientific and development aspects. The United States made an unprecedented investment into wartime research for the project, which was spread across over 30 sites in the U.S. and Canada. Scientific knowledge was centralized at a secret labotaory known as Los Alamos, previously a small ranch school near Santa Fe, New Mexico. Uranium appears in nature primarily in two isotopes: uranium-238 and uranium-235. When the nucleus of uranium-235 absorbs a neutron, it undergoes nuclear fission, splitting into two "fission products" and releasing energy and 2.5 neutrons on average. Uranium-238, on the other hand, absorbs neutrons and does . not fission, effectively putting a stop to any ongoing fission reaction. It was discovered that an atomic bomb based on uranium would need to be made of almost completely pure uranium-235 (at least 80% pure), or else the presence of uranium-238 would quickly curtail the nuclear chain reaction. The team of scientists working on the Manhattan Project immediately realized that one of the largest problems they would have to solve was how to remove uranium-235 from natural uranium, which was composed of 99.3% uranium-238. Two methods were developed during the wartime project, both of which took advantage of the fact that uranium-238 has a slightly greater atomic mass than uranium-235 : electromagnetic separation and gaseous diffusion- methods which separated isotopes based on their differing weights. Another secret site was erected at rural Oak Ridge, Tennessee, for the large-scale production and purification of the rare isotope. It was a massive investment: at the time, one of the Oak Ridge facilities (K-25) was the largest factory under one roof. The Oak Ridge site employed tens of thousands of employees at its peak, most of whom had no idea what they were working on.